Shaping the energy landscape toward renewable, carbon-free energy resources is a contemporary challenge that will require significant advancements in the development of catalysts/electrocatalysts for energy and chemical conversion processes. The goal of our research group is to design active, selective and stable heterogeneous catalysts and electrocatalysts for these processes. Specifically, we focus on the development of heterogeneous catalysts and electrocatalysts for electrochemical conversion and storage (i.e. fuel cells, electrolyzers, batteries) and cooperative/selective thermal catalysis for biomass upgrading, plastics upcycling and mitigation of greenhouse gasses. As an integral part of engineering catalytic/electrocatalytic structures, we implement a paradigm which involves a combination of controlled synthesis, advanced characterization, kinetic measurements and quantum chemical calculations to unearth the underlying mechanism that governs their catalytic performance for targeted reactions.
Research Projects
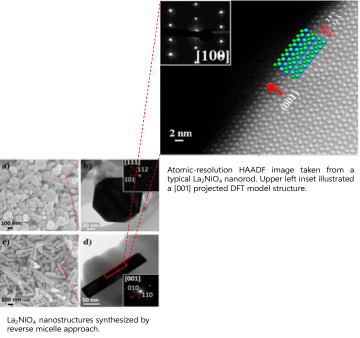
Engineering complex layered metal oxides for oxygen electrocatalysis.
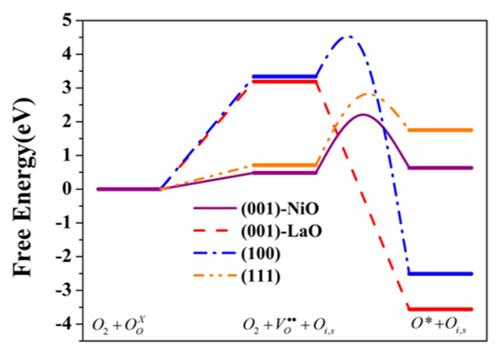
Oxygen electrocatalysis (ORR and OER) has attracted significant attention due to its critical role in the performance of important electrochemical energy conversion and storage devices, such as fuel cells, electrolyzers, and metal-air batteries. First-series Ruddlesden-Popper (R-P) oxides with a formula of A2BO4 have emerged as promising electrocatalysts for oxygen electrocatalysis due to their suitable mixed ionic and electronic conductivities. In this research project, we investigate the surface structure and composition as parameters to understand and tune the ORR/OER activity of these oxides. We utilize quantum chemical calculations combined with detailed characterization, controlled synthesis, and testing as effective ways for developing the fundamental knowledge at the molecular level required to guide the design of efficient nonstoichiometric, mixed metal oxides for oxygen electrocatalysis.
Related and Selected Publications:
- Dynamic Surface Reconstruction Unifies the Electrocatalytic Oxygen Evolution Performance of Nonstoichiometric Mixed Metal Oxides
Samira, S. ‡, Hong, J. ‡, Camayang, J. C.A., Sun, K., Hoffman, A.S., Bare, S.R., Nikolla, E.*, JACS Au, 2021. - Modulating Catalytic Properties of Targeted Cationic Metal Centers in Nonstoichiometric Mixed Metal Oxides for Electrochemical Oxygen Reduction
Samira, S., Camayang, J. C.A., Patel, K., Gu, X.K., Nikolla, E.*, ACS Energy Letters, 2021. - Electrochemical oxygen reduction on layered mixed metal oxides: Effect of B-site substitution
Samira, S. ‡, Camayang, J. C.A. ‡, Nacy, A.M. ‡, Diaz, M., Meira S.M., N., Nikolla, E.*, Journal of Electroanalytical Chemistry, 2019. - Design Strategies for Efficient Nonstoichiometric Mixed Metal Oxide Electrocatalysts: Correlating Measurable Oxide Properties to Electrocatalytic Performance
Samira, S., Gu, X. K. & Nikolla, E.*, ACS Catalysis, 2019. - Efficient Oxygen Electrocatalysis By Nanostructured Mixed Metal Oxides
Gu, X. K. ‡, Carneiro, J. S. ‡, Samira, S., Das, A., Ariyasingha, N., Nikolla, E.*, Journal of American Chemical Society, 2018. - Oxygen Sponges for Electrocatalysis: Oxygen Reduction/Evolution on Nonstoichiometric, Mixed Metal Oxides
Gu, X. K. ‡, Samira, S. ‡, & Nikolla, E.*, Chemistry of Materials, 2018. - Design of Ruddlesden–Popper Oxides with Optimal Surface Oxygen Exchange Properties for Oxygen Reduction and Evolution
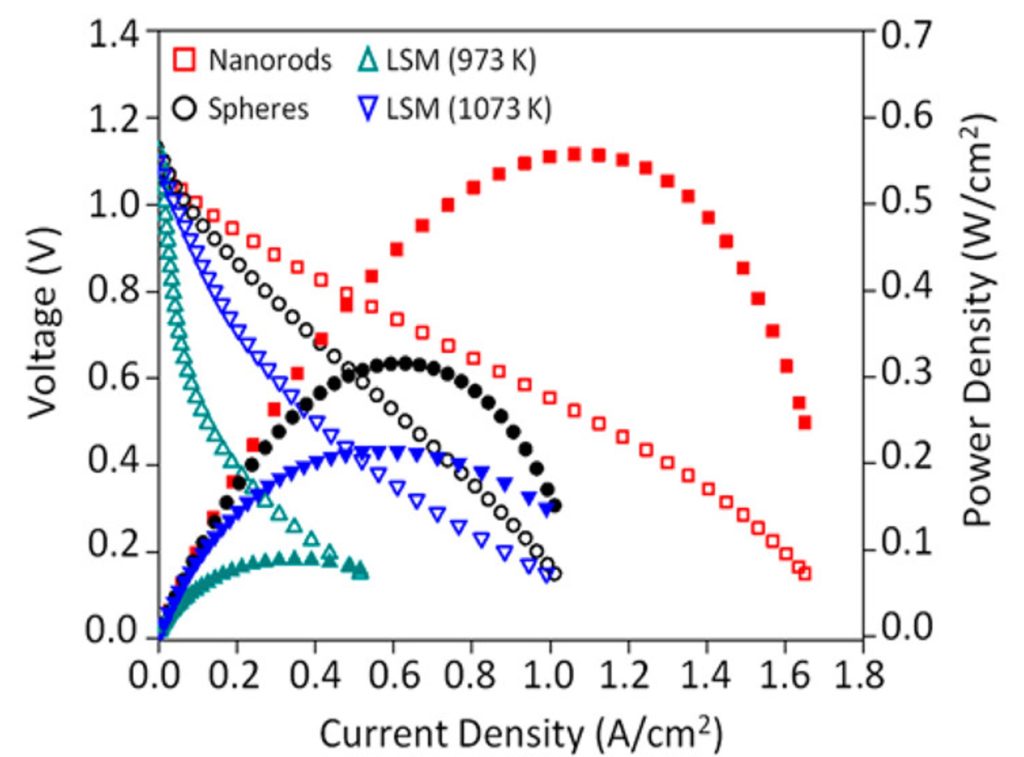
Gu, X. K. & Nikolla, E.*, ACS Catalysis, 2017. - Optimizing Cathode Materials for Intermediate-Temperature Solid Oxide Fuel Cells (SOFCs): Oxygen Reduction on Nanostructured Lanthanum Nickelate Oxides
Carneiro J. S. A., Brocca R. A., Lucena, M. L. R. S., Nikolla, E.*, Applied Catalysis B: Environmental, 2017. - Engineering Complex, Layered Metal Oxides: High-Performance Nickelate Oxide Nanostructures for Oxygen Exchange and Reduction
Ma X. ‡, Carneiro J. S. A. ‡, Gu X-K. ‡, Qin H., Xin H., Sun K., Nikolla E.*, ACS Catalysis, 2015. - Synthesis of shape-controlled La2NiO4+δ nanostructures and their anisotropic properties for oxygen diffusion
Ma X., Wang B., Xhafa E., Nikolla E.*, Chemical Communications, 2015.
‡ These authors contributed equally to this work
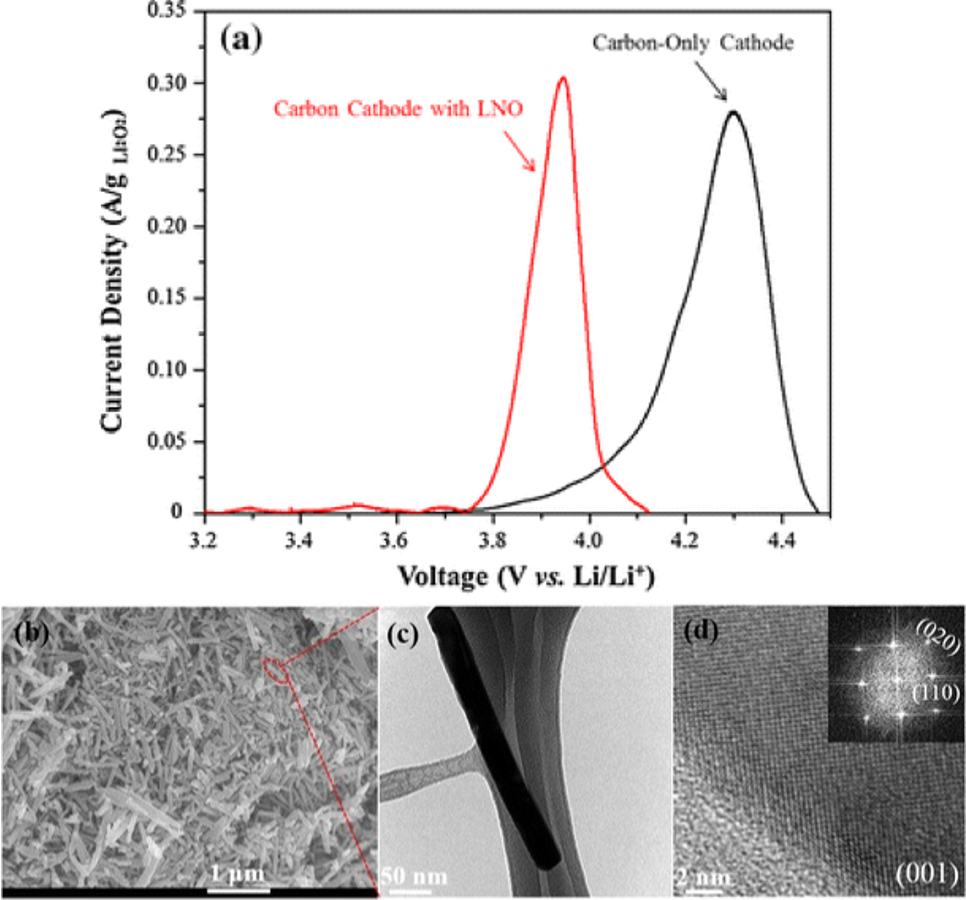
Tuning solid-solid interfacial catalysis in alkali metal-air battery cathodes.
The development of high-energy density batteries that enable electrification of the transportation sector remains a challenge. Metal-air batteries, especially the Li-air technology, have attracted significant interest over the last two decades due to their comparable energy density to that of gasoline. However, these systems are limited by large overpotential losses, during the molecular oxygen electrocatalysis resulting in low voltaic efficiencies. Our approach involves a combination of quantum theoretical calculations, controlled synthesis, kinetic studies, and thorough characterization to study and tune catalysis at the solid-solid interfaces of the battery cathodes where solid discharge products are formed and dissociated on solid electrocatalyst surfaces. The objective is to lower the cell overpotential losses by tuning the discharge product distribution.
Related and Selected Publications:
- Aprotic Alkali Metal-O2 Batteries: Role of Cathode-Surface Mediated Processes and Heterogeneous Catalysis
Samira, S., Deshpande, S., Greeley, J.P.*, Nikolla, E.*, ACS Energy Letters, 2021. - Non-Precious Metal Catalysts for Tuning Discharge Product Distribution at Solid-Solid Interfaces of Aprotic Li-O2 Batteries
Samira, S., Deshpande, S., Roberts, C.A., Nacy, A.M., Kubal, J., Matesić, K., Oesterling, O., Greeley, J.P.*, Nikolla, E.*, Chemistry of Materials, 2019. - Nanostructured Nickelate Oxides as Efficient and Stable Cathode Electrocatalysts for Li–O2 Batteries
Nacy A., Ma X., Nikolla E.*, Topics in Catalysis, 2015.
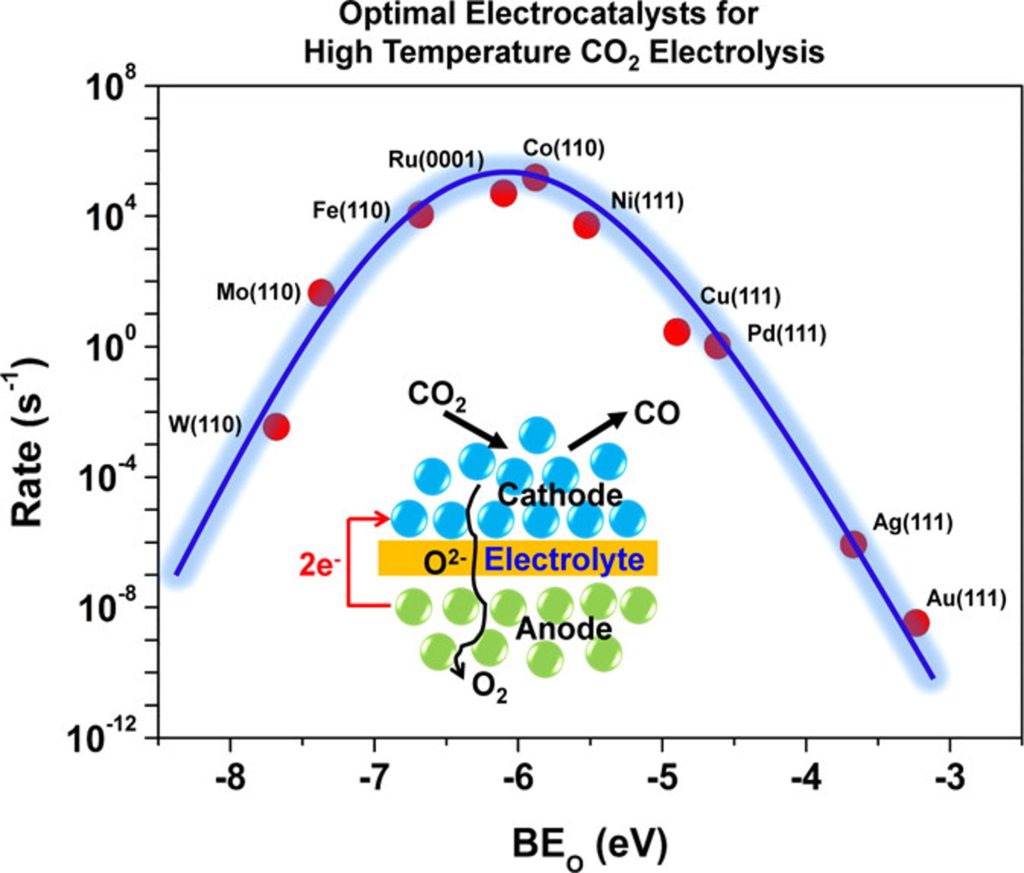
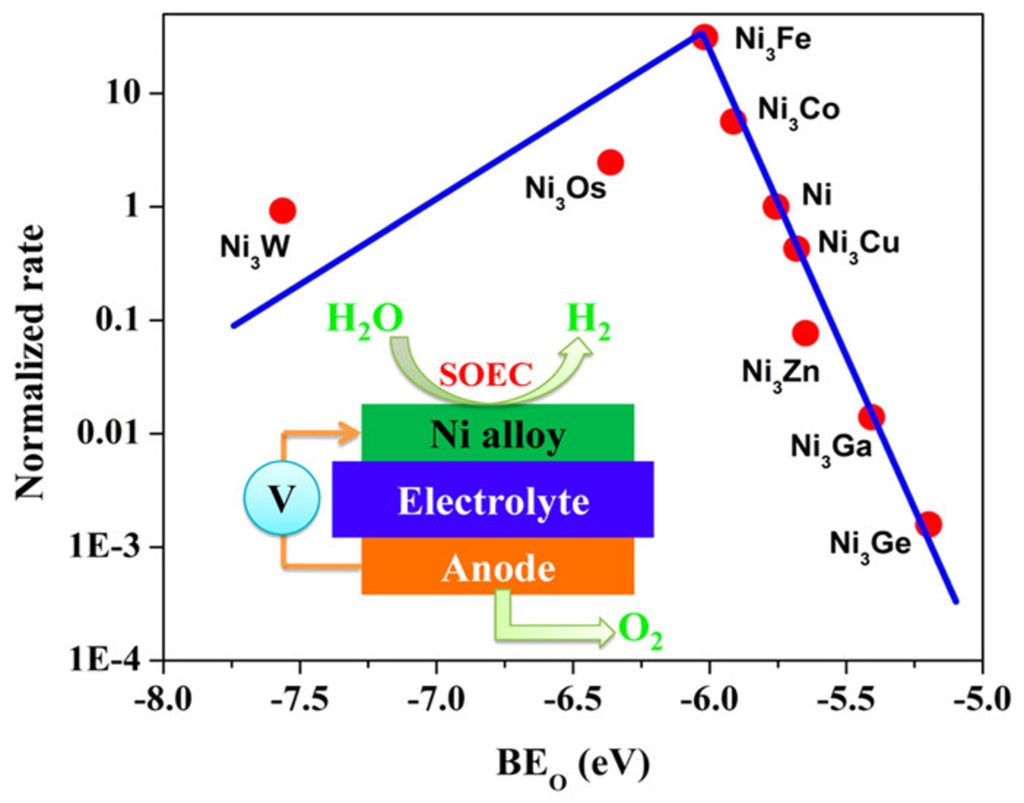
H2O splitting and CO2 reduction using solid oxide electrolyzers.
The interest in developing solid oxide electrolysis cells (SOECs) for electrochemical reduction of CO2 and water is steadily increasing as an approach to convert surplus energy from intermittent energy sources into chemical energy, while also mitigating CO2 emissions. The key challenges with this technology include high overpotential losses associated with the electrochemical reactions (i.e. oxygen evolution reactions (OER) and CO2 and H2O co-reduction) at the SOEC electrodes. This project aims to (1) investigate the optimization of catalytic activity of SOEC fuel electrode through the use of layered nickelate oxides as promising electrocatalysts for OER, and (2) assess the activity and stability of metal/metal-alloy based electrodes under conventional SOEC working conditions as electrocatalyst for and CO2 and H2O co-reduction. Our approach involves a combination of DFT calculations and microkinetic modeling with controlled electrochemical experiments to understand the chemical/electrochemical processes that govern H2O and CO2 co-electrolysis with the aim of identifying optimal electrocatalysts for this process.
Related and Selected Publications:
- Electrochemical reduction of CO2 on Metal-based Cathode Electrocatalysts of Solid Oxide Electrolysis Cells
Carneiro, J.S., Gu, X.K., Tezel, E., & Nikolla, E.*, Industrial & Engineering Chemistry Research, 2020. - First-Principles Study of High-Temperature CO2 Electrolysis on Transition Metal Electrocatalysts
Gu, X. K., Carneiro, J. S., & Nikolla, E.*, Industrial & Engineering Chemistry Research, 2017.
Special issue “Class of Influential Researchers”. - Heterogeneous Electrocatalysts for CO2 Reduction
Gu X-K. ‡, Carneiro J. S. A. ‡, Nikolla, E.*, Catalysis Vol. 29, Royal Society of Chemistry, 2017. - Fundamental Insights into High-Temperature Water Electrolysis Using Ni-Based Electrocatalysts
Gu X., Nikolla E.*, Journal of Physical Chemistry C., 2015.
‡ These authors contributed equally to this work
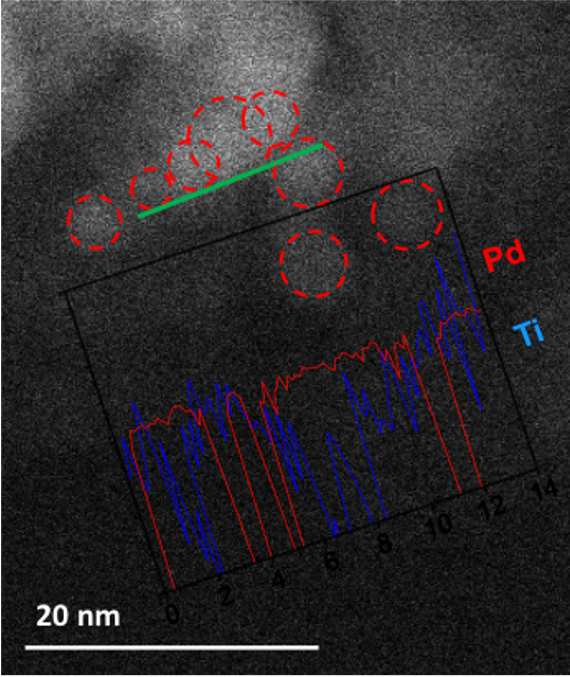
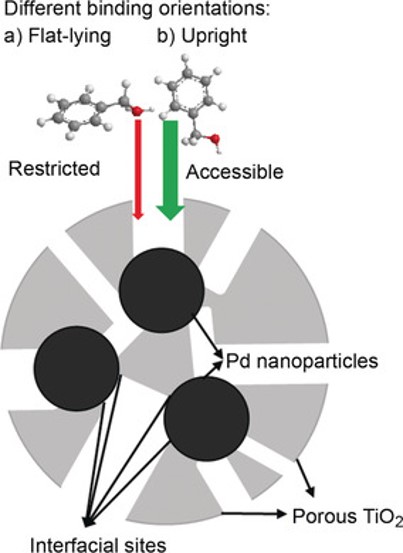
Design of multifunctional catalyst for selective catalysis.
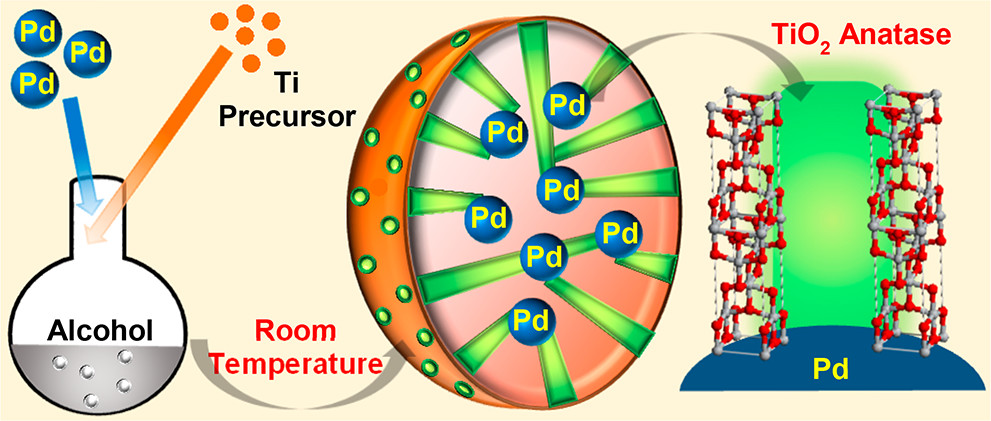
Metal-metal oxide catalysts have attracted significant attention in heterogeneous catalysis because of the interesting catalytic activity of the metal-metal oxide interface that can result in enhanced catalytic performance. In the project, our objective is to design “inverted” metal-metal oxide systems, in which metal oxide films are deposited on top of metallic substrates, in order to: (i) increase the density of metal-metal oxide interfacial sites; (ii) improve the chemical and thermal stability of small metal NPs by encapsulation; and (iii) provide spatially regulated delivery of reactants to the interfacial sites. One of the examples is the successful synthesis of encapsulated monodispersed Pd NPs in a porous TiO2 film with controllable pore size which leads to high selectivity toward hydrodeoxygenation of biomass-derived alcohols. (Zhang, J. *, Wang, B. *, Nikolla, E., & Medlin, J. W., Angewandte Chemie, 2017, 129, 6694) Catalyst selectivity was found to be a strong function of both the presence of Pd-TiO2 interfacial sites and the pore size of the TiO2 shell.
Related and Selected Publications:
- Selective C-O Bond Cleavage of Bio-based Organic Acids over Palladium Promoted MoOx/TiO2
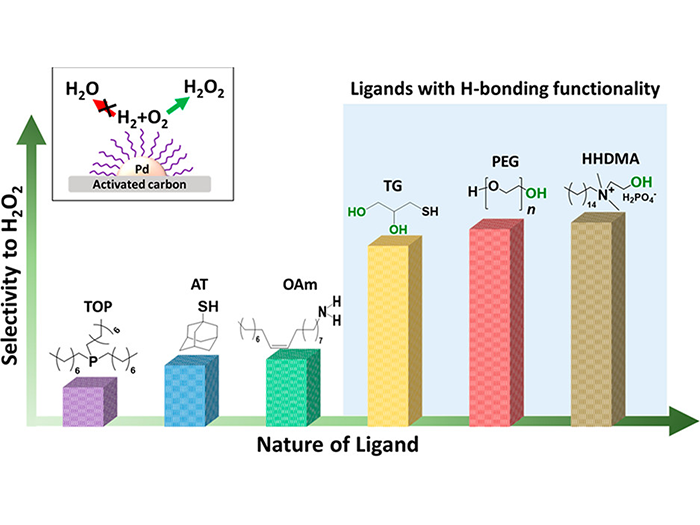
Nacy, A.M. ‡, Freitas de Lima e Freitas, L. ‡, Albarracin-Suazo, S., Ruiz-Valentin, G., Roberts, C.A., Nikolla, E.*, and Pagan-Torres, Y.J.* ChemCatChem, 2020. - Tunable Catalytic Performance of Palladium Nanoparticles for H2O2 Direct Synthesis via Surface-Bound Ligands
Freitas de Lima e Freitas, L. ‡, Puertolas, B. ‡, Zhang, J., Wang, B., Hoffman, A.S., Bare, S.R.,
Perez-Ramirez, J.*, Medlin, J.W.*, and Nikolla, E.*, ACS Catalysis, 2020. - 110th Anniversary: Fabrication of Inverted Pd@TiO2 Nanostructures for Selective Catalysis
Wang, B.‡, Zhang, J.‡, Herrera, L. P., Medlin, J. W.*, & Nikolla, E.*
Industrial & Engineering Chemistry Research, 2019. - Reaction Paths for Hydrodeoxygenation of Furfuryl Alcohol at TiO2/Pd Interfaces
Deo S., Medlin J. W., Nikolla E., Janik M. J.*, Journal of Catalysis, 2019. - Control of interfacial acid-metal catalysis with organic monolayers
Zhang, J.; Ellis, L. D.; Wang, B.; Dzara, M. J.; Sievers, C.; Pylypenko, S.; Nikolla, E.; Medlin, J. W.*
Nature Catalysis, 2018. - Multicomponent Catalysts: Limitations and Prospects
Kumar, G.; Nikolla, E.*; Linic, S.*; Medlin, J. W.*; Janik, M. J.*, ACS Catalysis, 2018. - Directing Reaction Pathways through Controlled Reactant Binding at Pd-TiO2 Interfaces
Zhang, J. ‡, Wang, B. ‡, Nikolla, E.*, & Medlin, J. W.*, Angewandte Chemie, 2017.
‡ These authors contributed equally to this work